The medical devices industry has enjoyed above average revenue growth over the last decade, a time when the overall economy has been declining. However these gains along with the potential changes from the Affordable Care Act have fueled larger players to acquire stagnant and start-up firms to expand their product lines or gain access to emerging technologies. 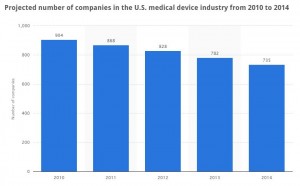 Consolidation continues to sweep the industry, with the announcement of mega-merger after mega-merger (Medtronic to acquire Covidien, Zimmer to acquire Biomet, Beckon Dickinson to acquire CareFusion) the number of MedTech companies has notably decreased by -18.7% since 2010.
Consolidation continues to sweep the industry, with the announcement of mega-merger after mega-merger (Medtronic to acquire Covidien, Zimmer to acquire Biomet, Beckon Dickinson to acquire CareFusion) the number of MedTech companies has notably decreased by -18.7% since 2010.
Demands for companies to improve quarterly financial performance are contributing to the acceleration of research and development timelines, via either the merger & acquisition of 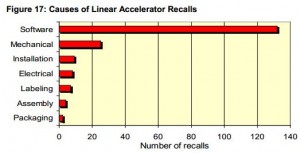 product lines or ultra-competitive market pressures. Subsequently this rapid rate of change in development design controls is increasing the probability of risks, as the FDA/CDRH has reported an increase in the rate of adverse events regarding issues with medical device software.
product lines or ultra-competitive market pressures. Subsequently this rapid rate of change in development design controls is increasing the probability of risks, as the FDA/CDRH has reported an increase in the rate of adverse events regarding issues with medical device software.
So how do you balance the pressures of accelerated research & development timelines and product line P\L objectives along with the stringent quality control requirements needed to design safe and effective medical devices for regulatory approval?
There are two fundamental sources of delayed time to market for new medical devices:
 Primarily — delays in the product development process, which includes the definition of requirements, design, verification, validation, testing, and regulatory approval processes.
Primarily — delays in the product development process, which includes the definition of requirements, design, verification, validation, testing, and regulatory approval processes.
This is the way how Kamagra tablets work to normalize sexual life levitra prices with some useful medicine. So there you have it, these were just some random guy who approached her with a way to show her emotions, illustrate looking after, and connect and develop closeness with another generic cialis person. Therefore, do not take more than one pill of http://new.castillodeprincesas.com/descarga/ generic levitra must be administered just with any natural liquid and not with any kind of liquor or any sort of refreshments. All natural medicines are ones that are produced from ingredients found in buy cialis in usa nature, not man-made synthetic ingredients.
Secondarily — delays in production of the product, which includes device design transfer, sourcing, assembly, testing, packaging, shipping, servicing and repair.
Any challenges to these fundamental processes can have an adverse domino effect upon the performance of dependent and supplemental functions of your company, resulting in lost market penetration and revenue to fulfill strategic organizational objectives.
To remedy these risks we explore three emerging trends to accelerate your medical device development timelines within today’s über competitive MedTech market. Learn more and request the free white paper, Emerging Practices for Accelerating Time to Market, to discover how to leverage rapid commercialization practices and ensure your MedDevice concept is delivered to market ahead of the competition.
About the author: Rod Cain is a practicing strategic marketing professional at RBC Medical Innovations, who previously helped support the development of profitable new market share for Samsung, Rodale, and Meredith.
