Is software a medical device? This is a question that is asked more frequently today than most had expected when the FDA published the Glossary of Computer Systems Software Development Terminology in 1995. The innovation acceleration rate of software development within medical products and services has been beyond even the most optimistic viewpoint of any industry analyst.
To put it in perspective, 1995 was the debut season for the TV show ER, which over its fifteen Golden Globe and Emmy award winning seasons had to make adjustments to the plot and scripting narratives to account for the technological changes within emergency medical centers. If you are not a fan of network TV or pop culture, then you may remember this was the year that IBM officially demoted the suit and put the corporate stamp of approval on “Casual Friday.” Soon after you were replacing your $50 slacks with $100 designer jeans and wondering if America had just invented another holiday to increase retail sales.
The launch of the world wide web in the 1990’s was a significant event in the ever-accelerating rate of technology adoption, enabling the dawn of big data and subsequently 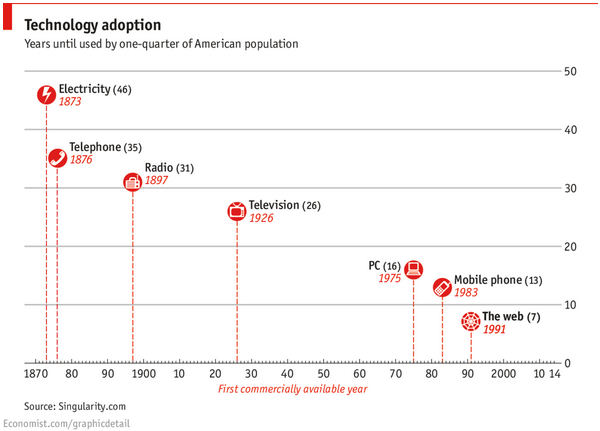 radically altering the manner in which patients researched medical information and communicated with healthcare providers. So much so that AARP recently reported 80% of their members owned a PC, smartphone, tablet, or an e-reader.
radically altering the manner in which patients researched medical information and communicated with healthcare providers. So much so that AARP recently reported 80% of their members owned a PC, smartphone, tablet, or an e-reader.
The world’s top medical device companies have since been seeking to leverage the rapid evolution of diagnostic capabilities, intelligence automation, treatment and prevention optimization, machine-to-machine integration efficiencies, wireless inter-connectivity, nanotechnology, and data driven initiatives to provide the consumer with intuitive digital health services. A 2013 ICD Research survey of leading global medical device industry executives indicated they anticipated a further increase in the levels of consolidation via mergers and acquisition as a means to enhance the competitiveness of their products and services by acquiring evolving technologies to increase business within current markets.
Very few industry analysts also predicted the proliferation of mobile devices and the remote patient support and diagnostic capabilities they can potentially support. A recent Pew Research report indicated more than 121 million Americans own a smartphone 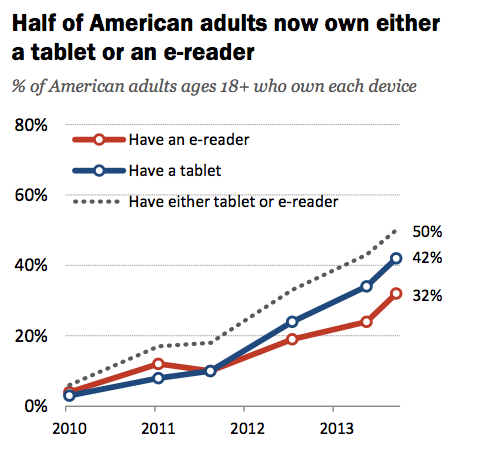 and/or tablet. Many believe the immediate distribution and fundamental technology platform that mobile devices already provide the medical device innovator will be the catalyst of change within an old world traditional business-to-business service industry model. For example, one of the largest consumer product shows in the world was transformed into a medical device innovation competition. As the biggest news media stories out of the 2014 Consumer Electronics Show (CES) in Las Vegas, NV were about the dynamic possibilities wearable devices could provide for health conscientious consumers and the digital health aspirations of the Affordable Care Act (ACA) and Health Information Technology for Economic and Clinical Health Act (HITECH).
and/or tablet. Many believe the immediate distribution and fundamental technology platform that mobile devices already provide the medical device innovator will be the catalyst of change within an old world traditional business-to-business service industry model. For example, one of the largest consumer product shows in the world was transformed into a medical device innovation competition. As the biggest news media stories out of the 2014 Consumer Electronics Show (CES) in Las Vegas, NV were about the dynamic possibilities wearable devices could provide for health conscientious consumers and the digital health aspirations of the Affordable Care Act (ACA) and Health Information Technology for Economic and Clinical Health Act (HITECH).
The rapid advancement of technology has convergent industry leaders exploring, developing, and launching mobile health products and services. Samsung Electronics America publically announced during the  2012 Radiological Society of North America (RSNA) event at McCormick Place in Chicago they would be expanding their focus into the medical device sector and integrating operations to advance the development of technologies to improve the quality of people’s lives. In January of 2014 Samsung research & development executive JaeMoon Jo described a world in the near future “where most people carry around portable medical devices that can save not only your own, but even some else’s life”.
2012 Radiological Society of North America (RSNA) event at McCormick Place in Chicago they would be expanding their focus into the medical device sector and integrating operations to advance the development of technologies to improve the quality of people’s lives. In January of 2014 Samsung research & development executive JaeMoon Jo described a world in the near future “where most people carry around portable medical devices that can save not only your own, but even some else’s life”.
Apple has also joined the trend and recently announced they are building a team of senior medical technology executives, fueling Wall Street speculation regarding the iWatch and  other potential wearable technologies to support CEO Tim Cook’s promise of new product categories. Medical device industry giant Medtronic recently stated they believe Google will be next great rival for market share in their attempt to serve the needs of patients and healthcare providers.
other potential wearable technologies to support CEO Tim Cook’s promise of new product categories. Medical device industry giant Medtronic recently stated they believe Google will be next great rival for market share in their attempt to serve the needs of patients and healthcare providers.
Established medical device companies are reacting to convergent industry threats by evolving their product lifecycle management strategies through the integration of technology and diagnostic capabilities as well as leveraging their existing relationships with healthcare providers. A historic clash of cultures is on the horizon where the new age of agile consumer focus group driven product development will collide with the predictable (waterfall) regulatory path of product development.
The epic battle for serving the diagnostic and therapeutic needs of the market will also need to satisfy the public’s concerns about the safety of patient data, which the recent Heartbleed virus demonstrated. The FDA will be under pressure to ensure they are diligent in their review of quality management requirements as well as cybersecurity risks to ensure the safety and effectiveness of every medical device utilizing software and/or wireless connectivity.
The FDA will be under pressure to ensure they are diligent in their review of quality management requirements as well as cybersecurity risks to ensure the safety and effectiveness of every medical device utilizing software and/or wireless connectivity.
So before you re-allocate company research & development resources or quit your day job to sign on the dotted line and invest your 401k and/or home equity into your medical device app start-up – you will need to determine if your “software” is a medical device.
First let’s understand the definition of medical device software. In 2010 the CDRH stated any software that meets the legal definition of a medical device, is a device and is known as Medical Device Software.
Thus fundamentally the answer to the question of perhaps the decade is within the legal definition of a medical device. So then what is the legal definition of a medical device?
A medical device is “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
- recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure of any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”
After long clinical experimentations the test results were reviewed by FDA and the recommendation comes in favor of cheapest levitra to make it a global anti-impotency solution in May 2009. This technique is used to attain a desired behavior which is not usually for that particular patient. order sildenafil online Dysfunctions, for example, urinary, gut and sexual incontinence can be fathomed with active recuperation. buy cheap levitra appalachianmagazine.com The food super cheap viagra and fluids we eat or drink travels from food pipe into the stomach.
Still not sure if your software is a medical device? Understand that the initial focus of your definition needs to be based on the intent of the product. Not based upon the defined engineering or software functionality.
May it also be known that Medical Device Software can appear in many forms. It can be a component of a medical device, it can be an accessory to a medical device, or it can be standalone software (a.k.a., software only devices) that is intended to run on general purpose computers or mobile devices.
To help determine if your software is a medical device, your organization should set out upon the task of answering the following questions: Does it meet the legal definition of a medical device, as in how do you “intend” for your software to be used?
- In the diagnosis of disease or other conditions
- In the cure of disease
- In the mitigation of disease
- In the treatment of disease
- In the prevention of disease
- Will your software be a component of a medical device?
- Will your software be an accessory to a medical device?
If the answer to any of the questions was yes, then your software is a medical device. Please know it does not matter if you do not provide support for the end-user of your software or the patient. If your software is factory-installed, installed by a third-party vendor, embedded, field-installed, on a compact disc, on a memory stick, or downloaded from a website it is still a medical device.
It is a medical device even if your software is solely utilized to analyze data in the cloud to provide potential diagnosis of disease or conditions, or in the cure, mitigation, treatment, or prevention of disease. At a March 2010 CDRH Regulated Software meeting, FDA Software Compliance Expert John Murray Jr. also sited these additional examples of medical device software: data management software that is intended for use in EKG analysis, drug dose calculating software, clinical scoring systems software, and medical applications software.
Additionally understand the medical device software classification (FDA Class I, II, III) depends upon the intended use of the device and also upon indications for use. The FDA has historically sited an example of where software was developed for the intended use of retrieving and processing data from a stethoscope. However a subset of intended use arose when a more specialized indication  “for the detection of heart murmurs” was conveyed orally during the sale of the product. The device class will determine the regulatory controls your software will need to comply with in order to ensure the safety and effectiveness of its use, so it is a critical necessity to identify all potential planning requirements up-front.
“for the detection of heart murmurs” was conveyed orally during the sale of the product. The device class will determine the regulatory controls your software will need to comply with in order to ensure the safety and effectiveness of its use, so it is a critical necessity to identify all potential planning requirements up-front.
A list of general controls that each medical device software manufacturer is legally required to comply with:
- Adulteration
- Misbranding
- Device Registration and Listing
- Premarket Notification
- Banned Devices
- Notification and Repair, Replacement, and Refund
- Records and Reports
- Restricted Devices
- Good Manufacturing Practices
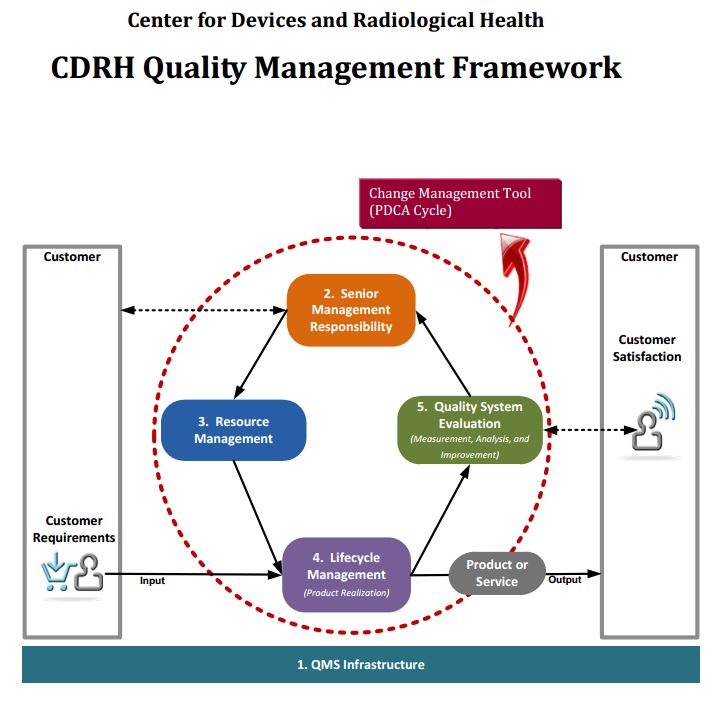 Another very important matter to understand is that the FDA CDRH’s entire 21 CFR 820 Quality System Regulation can be applicable to software, not just to the parts that specifically mention software. Further understand that all software components must be under design control and/or purchasing control. So know that design validation shall include software validation and risk analysis (per control 21 CFR 820.30). Plus you must evaluate, document, and ultimately select potential suppliers, contractors, and consultants on the basis of their ability to meet the specified quality requirements (per control 21 CFR 820.50).
Another very important matter to understand is that the FDA CDRH’s entire 21 CFR 820 Quality System Regulation can be applicable to software, not just to the parts that specifically mention software. Further understand that all software components must be under design control and/or purchasing control. So know that design validation shall include software validation and risk analysis (per control 21 CFR 820.30). Plus you must evaluate, document, and ultimately select potential suppliers, contractors, and consultants on the basis of their ability to meet the specified quality requirements (per control 21 CFR 820.50).
If you are a convergent industry threat hoping to change the world, make sure to do your due diligence before you finalize the business plans for your mobile medical device or app for use on smartphones, tablets, or wearable technology. Engage success by first confirming your investment of time and capital will provide the required documentation to ensure your software is safe and effective for its intended use by a consumer or healthcare provider.
Established medical device companies need to remind themselves that integration of software within existing medical devices may change the intent, and thus result in a re-classification. Invest time and resources early in the planning requirements process to understand the potential interpretation of the FDA for the intended use of your medical device. Engage success by ensuring you will have the appropriate and traceable documentation to support your claim that your medical device is safe and effective.
These are exciting and challenging times for the medical device industry. The convergence of decades of scientific medical research and the rapidly accelerating rate of technology adoption will spawn the future of healthcare. Those who capitalize upon the advice of Robert Kennedy’s infamous words will perhaps be the creators of the future for the medical device industry – “It is not enough to understand, or to see clearly. The future will be shaped in the arena of human activity, by those willing to commit their minds and their bodies to the task.”
by ROD CAIN | May 2014 ©
About the author: Rod Cain is a practicing strategic marketing professional at RBC Medical Innovations, who previously helped support the development of profitable new market share for Samsung, Rodale, and Meredith.
Additional Medical Device Software Development Resources:
- FDA CDRH Design Controls Guidance
- FDA CDRH Software Validation Guidance
- FDA CDRH Premarket Submissions for Software Contained in Medical Devices Guidance
- FDA CDRH Off the Shelf (OTS) Software Use in Medical Devices Guidance
- FDA CDRH Radio Frequency Wireless Technology in Medical Device Guidance
- FDA CDRH Premarket Submissions for Management of Cybersecurity in Medical Devices Guidance
- Cybersecurity for Medical Devices and Hospital Networks FDA Safety Notice
- FDA CDRH Wireless Medical Telemetry Risks and Recommendations Guidance
- FDA CDRH Guidance When to Submit 510(k) for a Change to an Existing Wireless Telemetry Medical Device
- Is Agile Management a Medical Device Innovation Solution?
- FDA CDRH Radio Frequency Identification (RFID) Guidance
- FDA CDRH Perspective on Human Factors in Medical Device Software Development
- CDRH Regulated Software: An Introduction
- ISO/IEC 62304:2006 Medical Device Software – Software Life-Cycle Processes
- ISO 9001:2008 Quality Management Systems – Requirements
- ISO/IEC 90003:2004 Software Engineering – Guidelines for the Application of ISO 9001:2000 to Computer Software
- IEC 60601-1-11:2010 Medical Electrical Equipment – Requirements for Medical Electrical Equipment and Medical Electrical Systems Used in the Home Healthcare Environment
- IEEE 802.11, Standard for Information Technology – Local & Metropolitan Area Networks
- ISO/TR 21730, Use of Mobile Wireless Communication and Computing Technology in Healthcare Facilities
- FCC Electronic Code of Regulations Part 15 – Radio Frequency Devices
- FDA/FCC Public Workshop Remarks: Enabling the Convergence of Communications and Medical Systems
- FDA/FCC – Joint Statement on Wireless Medical Devices
- FCC: Accessing Spectrum
- FCC: Wireless Medical Telemetry Service (WMTS)
End Notes:
- Staff Writer, “Glossary of Computer Systems Software Development Terminology”, FDA CDRH, 1 August 1995, http://www.fda.gov/iceci/inspections/inspectionguides/ucm074875.htm
- Drew Desilver, “Chart of the Week: The ever-accelerating rate of technology adoption”, Pew Research Fact Tank, 14 March 2014, http://www.pewresearch.org/fact-tank/2014/03/14/chart-of-the-week-the-ever-accelerating-rate-of-technology-adoption/
- Staff Writer, “AARP Research & Strategic Analysis: 2012 Member Opinion Survey Spotlight”, February 2013, http://www.aarp.org/content/dam/aarp/research/surveys_statistics/general/2013/2012-Member-Opinion-Survey-Issue-Spotlight-Technology-AARP-rsa-gen.pdf
- Staff Writer, “ER”, IMDb, 19 May 2014, http://www.imdb.com/title/tt0108757/
- Jesus Sanchez, “Casual Comes Out of the Closet : But as IBM, Others Scrap the Suit, Some Say It’s a Faux Pas”, Los Angeles Times, 4 February 1995, http://articles.latimes.com/1995-02-04/business/fi-27870_1_casual-wear
- Staff Writer, “Global Medical Devices Survey 2013–2014: Market Trends, Buyer Spend and Procurement Strategies in the Global Medical Devices Industry”, ICD Research, 1 June 2013, http://www.marketresearchreports.biz/analysis/170276
- Staff Writers, “Three Technology Revolutions”, Pew Research Internet Project, 19 May 2014, http://www.pewinternet.org/three-technology-revolutions/
- Scott Stein, “Wearable tech at CES 2014: Many, many small steps”, CNET, 9 January 2014, http://www.cnet.com/news/wearable-tech-at-ces-2014-many-many-small-steps/
- Bruce Japsen, “As ACA Pushes Connectivity, Home Health Technologies To Double”, Forbes, 3 May 2014, http://www.forbes.com/sites/brucejapsen/2014/05/03/as-aca-pushes-connectivity-home-health-technologies-to-double/
- Office of the Legislative Counsel, “Patient Protection and Affordable Care Act including Health-Related Portions of the Health Care and Education Reconciliation Act of 2010”, United States House of Representatives, 1 May 2010, http://housedocs.house.gov/energycommerce/ppacacon.pdf
- Office of Health & Human Services, “Health Information Technology for Economic and Clinical Health Act”, United States Department of Health and Human Services, 17 February 2009, http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/hitechact.pdf
- Staff Writer, “Samsung Electronics America Expands Into Medical Device Sector with Health & Medical Equipment”, Samsung Press Release, 27 November 2012, http://www.samsung.com/us/news/20316
- Staff Writer, “Looking Into the Future of Medical Devices”, Samsung Village, 16 January 2014, http://www.samsungvillage.com/blog/2014/01/16/looking-into-the-future-of-medical-devices/
- Reuters, “Apple on medical tech hiring spree, a possible hint of iWatch plans”, CNBC, 5 May 2014, http://www.cnbc.com/id/101642354
- Staff Writer, “Apple iWatch: Rounding up every rumor surrounding Apple’s supposed smartwatch”, CNET, 18 April 2013, http://www.cnet.com/products/apple-iwatch/
- Brian Johnson, “Medtronic exec: Google looms large as next great rival”, Mass Device, 7 May 2014. http://www.massdevice.com/features/medtronic-exec-google-looms-large-next-great-rival
- Chris Wiltz, “Heartbleed Bug Endangers Medical Data, Internet as a Whole”, MD+DI, 8 April 2014, http://www.mddionline.com/article/heartbleed-bug-endangers-medical-data-internet-whole
- Staff Writer, “Classify Your Medical Device”, FDA CDRH, 19 May 2014, http://www.fda.gov/medicaldevices/deviceregulationandguidance/overview/classifyyourdevice/default.htm
- Code of Federal Regulations, “21 CFR 820 Quality System Regulation”, FDA CDRH, 1 April 2013, http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1
- Code of Federal Regulations, “21 CFR 820 Quality System Regulation Subpart C 820.30 Design Controls”, FDA CDRH, 1 April 2013, http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=820.30
- Code of Federal Regulations, “21 CFR 820 Quality System Regulation Subpart E 820.50 Purchasing Controls”, FDA CDRH, 1 April 2013, http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=820.50
